Life science made better with real-world data
Life Science
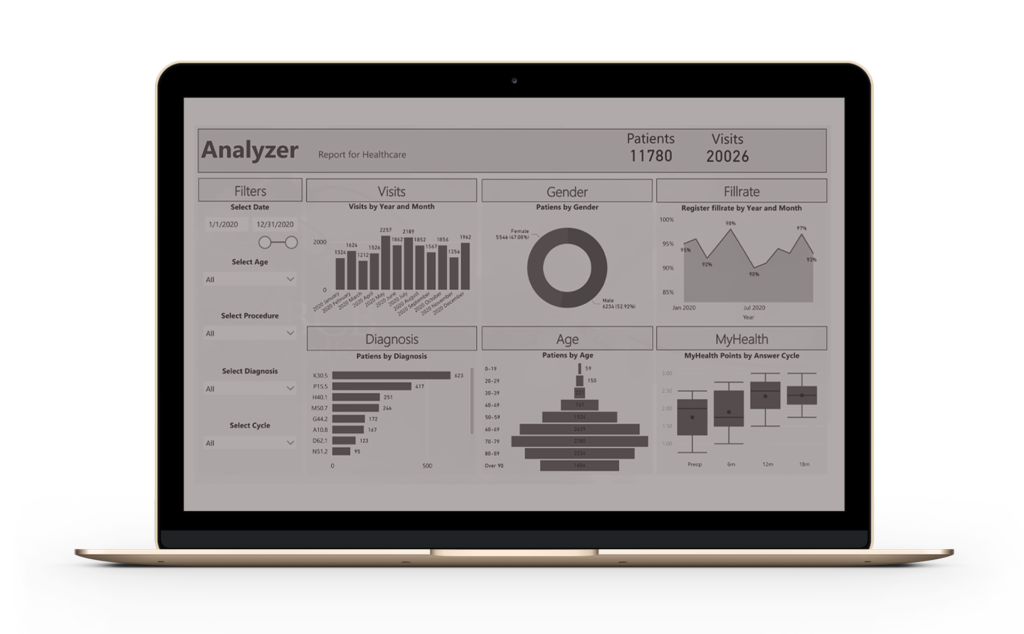
We combine, process, and analyse real-world clinical data and offer valuable insights in an easy-to-use, visualised format for healthcare providers, pharmaceutical industry, and researchers.
We gather clinical data from our disease-specific clinical information management systems (CIMS) and through our integrated proprietary platform. From that real-world data, our BCB Data Science Engine produces regulator-worthy, clinical research-grade data and offers insights for fast-track clinical decisions and trials. Our solutions also enable national and regional benchmarking with consistent disease-specific indicators relating to treatment results and help to identify key areas in need of development.
High quality data and secure data transfer
Thanks to our Data analytics, BCB Data Science Engine is an essential, single system tool for improved patient management, reduced healthcare costs, and research advancements. The quality of the data is ensured by our clinical CIMS, designed and continuously maintained by our highly skilful Engineering team.
The data collected from our disease-specific clinical information management systems can be transferred to hospital data storages and data lakes using the safe and secure BCB Content Broker service.
Working with synthetic data
BCB medical is a high tech, trustworthy and solid partner. We have broad range of experience of artificial intelligence algorithms and synthetic data production.
As a part of our synthetic data operations, we work actively in Privasa collaboration project. The aim of the project is to develop artificial intelligence methods that can replace sensitive data from real patients with secure de-identified synthetic data based on the real patients. The end result is material that can be processed without compromising the privacy of individuals.
Synthetic data is used as a source for various projects, for instance, for digital twins that can be used for healthcare process testing, clinical analysis, and more cost-effective precision medicine development. Synthetic data can also be one of the solutions in the current data sharing challenge.
Data harmonization
We are reaching towards the optimal usability of the collected clinical data. To be able to use the data in larger studies and in global research and benchmarking, the data needs to be in structured and standardised form.
BCB Medical is working as an active member in EHDEN, the European Health Data & Evidence Network. EHDEN aims to develop a common, standardized data model for the EU and BCB Medical works for this collective mission by standardising its own data to meet the EHDEN model criteria.
iCRF
Conduct more cost-effective studies by using iCRF, our next generation electronic CRF with integrated data collection. With automatic data collection you avoid duplicate data entry, which means saving time and avoiding errors. The iCRF can be used with our decision support system RealQ or customized to suit clinical studies in other settings.
Utilising the data for research and studies
Analytics of medical data requires high quality, relevant and reliable data. The data collected through BCB Medical’s Clinical Information Management Systems can be utilised in various ways, for example in medical research and in the publication of scientific articles.
Here you can find a list of studies and publications that have applied data collection methods that meet scientific research criteria and are ethically sustainable. The publications are utilising patient-specific data contained and produced by BCB Medical’s CIMS used in daily treatments and operations in hospitals.